Abstract
Introduction: The JAK/STAT pathway is frequently activated in peripheral T-cell lymphomas (PTCLs) and cutaneous T cell lymphomas (CTCLs) however the utility of JAK inhibition as a therapeutic strategy in these diseases is not known. We hypothesize that JAK/STAT pathway alteration (defined by elevated phospho-STAT3 (pSTAT3) expression or presence of JAK or STAT mutations) will predict for sensitivity to JAK inhibition in PTCL and CTCL. We are conducting a phase II study of ruxolitinib, a JAK1/2 inhibitor, in which we assess its efficacy in a cohort of patients (pts) with PTCL and CTCL enriched for JAK/STAT pathway alterations.
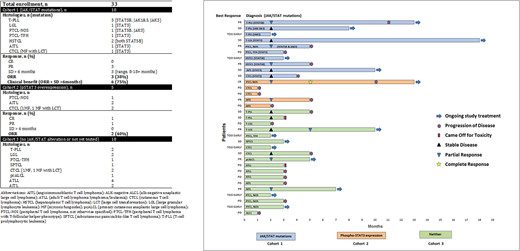
Methods: This is an investigator-initiated multi-center phase II study evaluating the efficacy of ruxolitinib 20mg twice daily in PTCL and CTCL. Pts with relapsed or refractory (rel/ref) PTCL or CTCL following at least 1 systemic therapy are eligible to enroll onto one of three of the following cohorts: Cohort 1: Disease determined to have JAK or STAT mutations. Cohort 2: Disease with functional evidence of JAK/STAT activation (defined as ≥ 30% pSTAT3 expression by immunohistochemistry). Cohort 3: Disease does not meet criteria for cohort 1 or 2. Pts initially enrolled onto cohort 3 and determined to have JAK/STAT mutations or functional JAK/STAT activation after enrollment are moved to cohorts 1 or 2. Cohorts 1, 2, and 3 are enrolling up to 17, 17 and 18 pts respectively by Simon 2-stage design. Pts receive treatment until progression and are assessed for response to therapy after cycles 2, 5 and every three cycles thereafter.
Results: The first stage has been completed for cohorts 1 and 3 and 33 out of planned 52 pts enrolled to date. Details regarding histology, mutations, and treatment course appear in the table and figure. For Cohort 1, 10 out of planned 17 pts enrolled. Mutations involved JAK1 (1), JAK3 (3), STAT3 (4), and STAT5B (4). Out of 8 evaluable pts, overall response rate (ORR) was 38% with 3 partial responses (PR). In addition, 3 pts have ongoing stable disease (SD) lasting 8-18 months. Altogether, 75% achieved clinical benefit (objective response or SD >6 months). For Cohort 2, 5 out of planned 17 pts enrolled. Among the 5 evaluable pts, ORR was 40% with 1 complete response (CR) and 1 PR. For Cohort 3, 18 pts out of planned 18 pts enrolled. Out of 14 evaluable pts, ORR was 21% with 3 PRs.
Grade 3 or higher drug-related adverse events (AEs) observed among the 33 pts enrolled included anemia (4), neutropenia (7), thrombocytopenia (2), and lymphopenia (7). Treatment related serious adverse events (SAEs) included 1 episode each of HSV-1 stomatitis, spontaneous bacterial peritonitis, febrile neutropenia, and herpes zoster.
Conclusion: Responses observed across all three cohorts of pts with PTCL and CTCL with a trend towards higher rates and more durable responses among pts with JAK/STAT alterations. Efficacy of ruxolitinib in PTCL and CTCL provides proof of concept that JAK/STAT activation is a viable target in T-cell lymphomas. Enrollment onto cohorts 1 and 2 and assessment for JAK/STAT alternations among pts in cohort 3 continues.
Moskowitz:Incyte: Research Funding; ADC Therapeutics: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Bristol Myers-Squibb: Consultancy, Research Funding; Merck: Research Funding; Takeda: Honoraria. Jacobsen:Merck: Consultancy; AstraZeneca: Consultancy; Seattle Genetics: Consultancy. Weinstock:Travera: Equity Ownership; Astra Zeneca, JAX, Samumed, Regeneron, Sun Pharma, Prescient: Patents & Royalties; Novartis, Dragonfly, Travera, DxTerity, Travera: Consultancy; Novartis, Astra Zeneca, Abbvie, Aileron, Surface Oncology, Daiichi Sankyo: Research Funding; Novartis: Consultancy, Research Funding; Genentech/Roche, Monsanto: Consultancy. Horwitz:Aileron Therapeutics: Consultancy, Research Funding; Portola: Consultancy; Celgene: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; Spectrum: Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Innate Pharma: Consultancy; Trillium: Consultancy; Forty Seven: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Mundipharma: Consultancy; Millennium/Takeda: Consultancy, Research Funding; Corvus: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal